On April 10, 2025, the U.S. Food and Drug Administration (FDA) announced that it plans to move away from animal testing in the development of some drugs. The FDA stated that the major purpose of this practice is to speed up the process of new drug development and improve overall safety of the drugs. By decreasing animal testing, pharmaceutical companies can lower research and development costs and, ultimately, drug prices.
FDA Commissioner Dr. Martin A. Makary said, “By leveraging AI-based computational modeling, human organ model-based lab testing, and real-world human data, we can get safer treatments to patients faster and more reliably, while also reducing R&D costs and drug prices.” The debate over the effectiveness of animal testing has lasted for a long time. Some scholars believe that animal experiments are unethical and that toxicology research results may not apply to human clinical trials. While some experts believe that, currently, no technology can immediately replace animal testing.
The FDA’s shift away from animal testing builds on decades of efforts to promote alternative methods. As early as 1985, the FDA Toxicology Research Department began to promote in vitro tests (such as cell culture) as a supplement to animal experiments based on the “3R principle” (i.e., Replacement, Reduction, and Refinement).
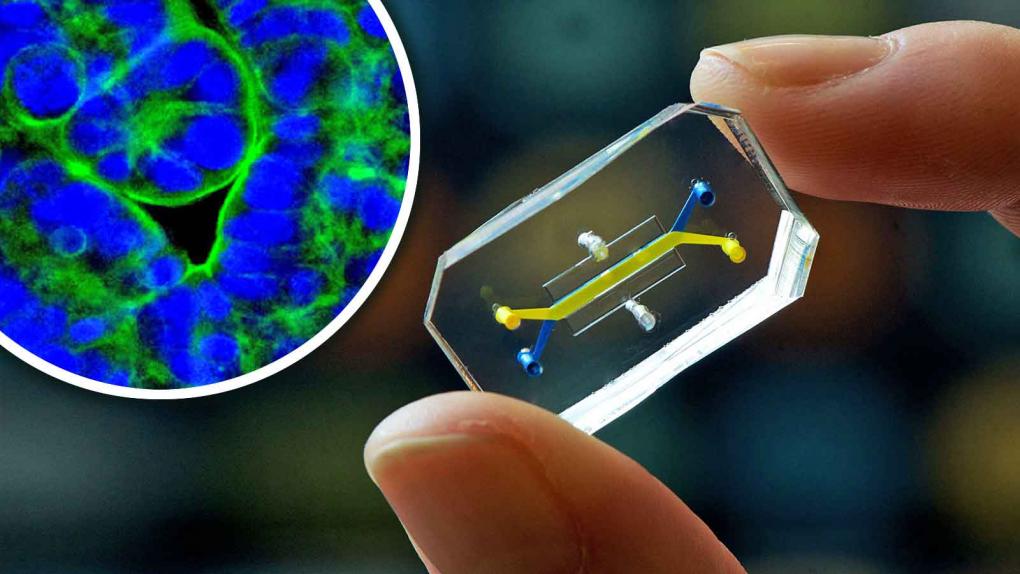
In 2017, the FDA released the “Predictive Toxicology Roadmap”, encouraging the use of computer models and in vitro methods to reduce the use of animal testing. On September 29, 2022, the U.S. Senate passed the FDA Modernization Act 2.0, which abolished federal mandatory requirements for animal testing of new drugs and allowed the use of alternative animal testing methods in drug development and production to study drug effectiveness and safety. On December 12, 2024, the U.S. Senate unanimously passed the revised “FDA Modernization Act 3.0”, which encourages the use of new methodologies, such as organoids and organ chips, to replace animal testing and promotes pharmaceutical companies toward “animal-free testing”.
Nevertheless, these new technologies, such as organ chips, organoids, and in vitro cell models, still have some unresolved risks. Organoids, for instance, cannot fully replicate key functions of the human body, including blood circulation and immune responses. Moreover, the lack of standardization and verification systems means that results can vary significantly across different laboratories, making it difficult to ensure consistent quality and reliability.
Alternatives to AI-based computational models are also often mentioned. However, Matthew R. Bailey, chairman of NABR, believes that although AI is expected to accelerate the development of many research fields, there are some problems. For example, it relies heavily on the extraction of existing data, and unknown variables may pose the greatest risk to patients. At present, no AI model can fully replicate all the unknown areas of a complete biological system. For this reason, animal research remains indispensable.
Therefore, the more realistic approach at present is to use alternative methods as a supplement: they can screen and predict early in the R&D process, decrease the number of ineffective or high-risk drug candidates entering animal testing, and thus reduce the use of animals. At the critical stage, animal models will remain one of the regulatory-approved safeguards. However, as these technologies mature, the FDA’s commitment to innovation could not only transform drug development but also signal a turning point in ethical science, paving the way for more humane and effective treatments in the future.
Sources:
Office (2025). FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs. [online] U.S. Food and Drug Administration. Available at: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs [Accessed 28 Apr. 2025].
Buntz, B. (2025). Xaira taps scGPT pioneer Bo Wang to lead biomedical AI. [online] Drug Discovery and Development. Available at: https://www.drugdiscoverytrends.com/how-scgpt-pioneer-bo-wang-ph-d-and-xairas-1b-war-chest-aim-to-build-a-virtual-cell/ [Accessed 28 Apr. 2025].
Qian, L., Dong, Z. and Guo, T. (2025). Grow AI virtual cells: three data pillars and closed-loop learning. Cell Research. [online] doi:https://doi.org/10.1038/s41422-025-01101-y.
Bunne, C., Roohani, Y., Rosen, Y., Gupta, A., Zhang, X., Roed, M., Alexandrov, T., AlQuraishi, M., Brennan, P., Burkhardt, D.B., Califano, A., Cool, J., Dernburg, A.F., Ewing, K., Fox, E.B., Haury, M., Herr, A.E., Horvitz, E., Hsu, P.D. and Jain, V. (2024). How to build the virtual cell with artificial intelligence: Priorities and opportunities. Cell, [online] 187(25), pp.7045–7063. doi:https://doi.org/10.1016/j.cell.2024.11.015.